What is uPVC and Where did it come from?
A Brief History of PVC, How and Whom discovered it and its final breakthrough for manufacturing.
Henri Victor Regnault
In 1838 French physicist Henri Victor Regnault accidentally discovered PVC for the first time. Regnault was highly regarded for his investigations into thermodynamics. During one of his investigations Regnault was experimenting with heating ethylene chloride (dutch oil). He was attempting to decompose the oil with potassium hydroxide, unknowingly, he obtained the monomer of vinyl chloride in the form of a gas in his flask. By chance he accidentally left the flask filled with gas on a shelf in the sun and over time a hard white substance formed, unfortunately, he couldn’t find a practical use for it and never took his discovery any further or patented it.


Eugene Baumann
34 years later the second discovery was made, this time in 1872 by German chemist Eugen Baumann. He accidentally synthesized PVC in his laboratory after leaving a flask of vinyl chloride standing in sunlight (similar to what Regnault had done 34 years earlier). Once again, the resulting polymer was a hard, solid white lump. Similarly, no use could be found for this strange, hard white substance and it was largely ignored and still unpatented.
Friedrich Klatte
Moving forward to 1913, Friedrich Klatte, another German chemist, was the first to realise the potential of vinyl so he applied for (and received) the patent for it. However, despite his best efforts, he couldn’t find a viable commercial use for the substance as it was very brittle and difficult to work with. At the time, his patent didn’t bear any fruit for him.


Waldo Semon
Up until the 1920s and despite PVC being well documented, it didn’t seem to have much practical use in the real world. However, Waldo Semon decided to take a bit of time out from his everyday duties as a chemist at BF Goodrich and began experimenting with PVC. Semon was endeavouring to solve a problem the company was having when coating metal with rubber and he was also investigating a cheaper synthetic replacement for natural rubber.
What Semon discovered “by accident” according to him, was that if he heated the PVC polymer in a solvent that had a high boiling point, he ended up with a gel-like substance that once cooled, was elastic in nature. This was a huge breakthrough compared to its hard, brittle and inflexible predecessor. Realising the significance of what he’d done, he turned his full attention to his new vinyl breakthrough. After further refinements, he found that not only could it be reproduced cheaply, it was also more resilient, flexible, versatile and durable than natural rubber and could also be poured into moulds to make pretty much any shape imaginable.
PVC production is the result of a complex chemical process involving a very long value chain.
In simple terms - Chlorine + Ethylene = PVC
How do we get Chlorine and Ethylene?

Chlorine Manufacture
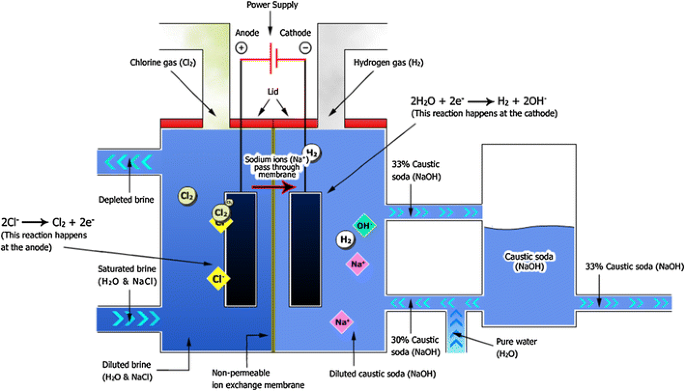
The production of PVC raw material requires two raw products, one of which is Chlorine. Chlorine’s origins stem from the Caustic Soda manufacturing industry. Caustic Soda production utilises an abundant natural resource in Salt or Sodium Chloride, Sodium Chloride is transformed into brine, an aqueous solution that undergoes a process known as Chlor-alkali or electrolysis.
This process produces chlorine, hydrogen, and sodium hydroxide (also known as caustic soda). Chlor-alkali is mainly used for caustic soda production, which is widely used in various industries across the globe. Chlorine is a byproduct of this process and has many other applications besides PVC manufacturing. Around 40% of the chlorine produced globally is specifically used for PVC production.
Ethylene Manufacture
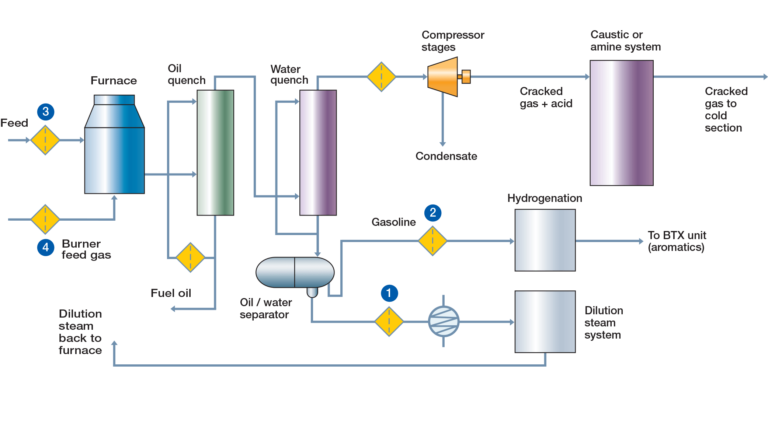
The second raw material is Ethylene. Ethylene is typically a byproduct of the petrochemical industry, generated through the process of thermal cracking of naphtha or natural gas. However, there is an alternative method of manufacturing PVC that involves using Acetylene sourced from Coal, which is mainly carried out in Asia, in particular China.
The latest technology is allowing the production of Bio-Ethylene from natural products like sugar cane. Currently, Ethylene is the primary raw material used in the production of PVC in Europe and North America, but efforts are underway to shift towards Bio products and reduce our dependence on fossil fuels.
“Two by-products of other industries that had little use, would lead to the discovery of a substance that has become the world’s third-most widely used synthetic polymer. When PVC Resin is converted it produces the most diverse characteristics and range of applications.”
How do we get PVC Raw Material?

The Process of PVC Resin
The PVC production process combines ethylene with chlorine to produce an intermediate product known as EDC (ethylene dichloride or 1,2-dichloroethane). EDC is transformed into vinyl chloride through the process of polymerisation, which involves linking together the simplest units of vinyl chloride called the monomers to form long molecular chains called polymers, this result produces polyvinyl chloride (PVC) in the form of a white powder. The PVC powder or what we will call unmodified PVC is the basis for a wide range of PVC applications, each application uses a combination of other ingredients and a variety of techniques to manufacture the final products that we see in everyday use.

Why is PVC Important?
PVC is one of the world’s oldest and most researched polymers, its chemistry has been understood since the end of the 19th century. PVC is produced mainly via ethylene and chlorine through the polymerisation of vinyl chloride monomer (vinyl chloride). The high economic significance of PVC comes from its low production costs as well as its exceptional characteristics, the most important of which include a long lifespan and attractive mechanical, electrical, chemical and thermal resistance properties.
In its basic form, unmodified PVC is a hard and brittle compound. However, due to its polar nature, it is often converted by incorporating a wide range of additives, these additives transform PVC into many compounds which is the reason why PVC is known as the holy grail of plastics, due to its amazing versatility and substantial range of applications.
PVC Applications in Everyday Life
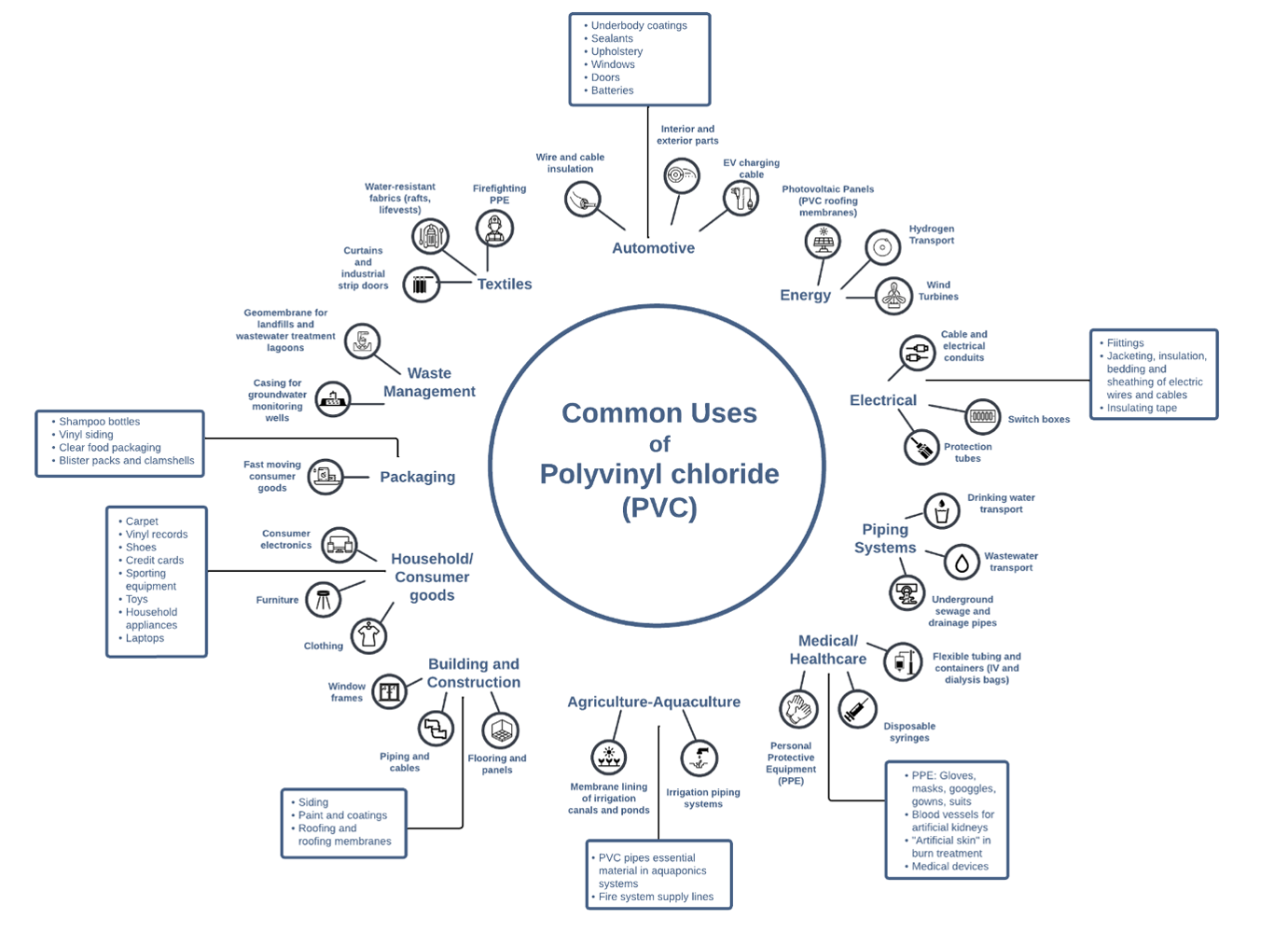
PVC Resin requires converting into a compound by incorporating various additives for manufacturing, conversion produces two distinct families, they either become modified PVC or uPVC.
What is the difference between PVC and uPVC?
We can use expressions like "chalk and cheese" to summarise the relationship between PVC and uPVC.
uPVC stands for Unplasticized Polyvinyl Chloride, while PVC stands for Polyvinyl Chloride, both originate from the inert unmodified PVC Resin. However the similarities stop here, they are immensely different in their composition and manufacturing processes.
uPVC is a rigid form of PVC that does not contain plasticisers and various additives like PVC, making it more durable and suitable for external applications such as windows and doors.
PVC is like “Cheese”
PVC is a material that is commonly used in some plumbing, electrical insulation, and other everyday applications. It is softer, more flexible, and malleable compared to other materials. In recent history, it has been acknowledged that PVC manufactured products have contained some additives and compounds, such as lead, cadmium, and mercury, as well as BPAs and plasticizers that are known to be harmful to people’s health and cause environmental problems. Despite its usefulness, the use of compounds such as BPAs and Phthalates (Plasticisers DEHP, DPB) in PVC manufacturing is now restricted.
Aside from the restricted products, the most significant issue for PVC manufacturing relates to the formation of bonds between the additives and PVC Polymers, a specific problem commonly known as “Plasticiser Migration” which occurs when the Plasticisers used in PVC product manufacturing remain unbound to the PVC polymers, Plasticisers are highly important to PVC manufacturing as they allow the plastics to become flexible and malleable which give PVC its amazing characteristics, unfortunately the occurance of unbound additives mean they can migrate, evaporate, or leach-out of the products during use, in their life-cycle or over time. Since most PVCs are considered specific-use or single-use items, they are now not recycled in New Zealand and end up as refuse waste, causing environmental problems.
uPVC is like “Chalk”
The process to produce uPVC profiles is highly advantageous over PVC, Eco-friendly additives and compounds are utilised during manufacturing, the actual process of conversion to uPVC is actually about improving on the PVC Resins characteristics, and performance for manufacturing, whereas modified PVC is actually being completely modified to achieve the desired results, another advantage for uPVC is the formation of bonds during conversion between the additives and polymers, meaning that the additives are locked in the chemical chain, unlike modified PVC cannot migrate, leach or evaporate out of the product.
In the context of windows, the conversion process improves uPVC’s strength, durability, energy efficiency and low maintenance, uPVC products are sustainable and eco-friendly, they do not use the same additives or plasticisers to produce the final product, which ensures that uPVC can have a sustainable life cycle and be reused again and again.
What is uPVC used for?
Unplasticised Polyvinyl Chloride is a low-maintenance material used as a substitute for painted wood and metal products, it is lightweight, strong and low maintenance making it a popular choice for homeowners.
uPVC has many uses which include windows and doors, fascia, cladding and decking. The same material has almost entirely replaced the use of copper, galvanised iron and concrete for plumbing and drainage, uPVC is widely used for waste pipes, drainpipes, guttering and downpipes.
The manufacturing of uPVC is free of BPAs, Plasticisers, and other elements or chemicals considered a health or environmental risk.

Manufacturing of uPVC Window and Door Profiles
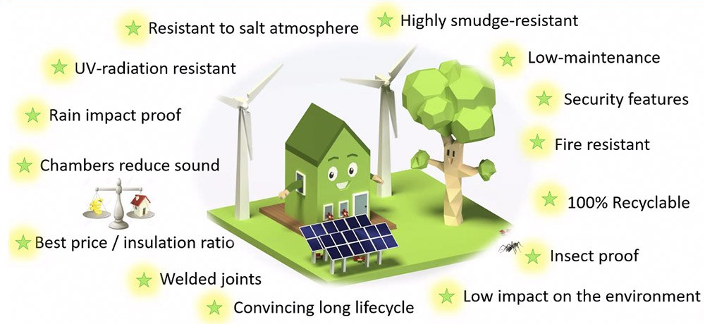
When manufacturing uPVC profiles, the resulting product possesses some remarkable qualities
Unmodified uPVC powder, in itself, is an inert and non-conductive substance that has limited use. The introduction of compounds and additives revolutionized the game and the same applies to uPVC. Specific additives are necessary to achieve the desired specifications, which ultimately lead to the production of high-quality profiles.
uPVC is a robust, durable, weather-resistant, non-conductive and sound-reducing, thermally efficient, UV-resistant, easy to clean, and requires no painting
The first step in the manufacturing process is selecting high-quality resins that contribute to the formation of a resin mix. The next step is adding titanium dioxide and calcium carbonate to the resin mix during the extrusion process. Large-scale automatic mixing equipment are used to ensure the mix is churned correctly. Once the molten mix is ready to become a profile, the mix is cast into a die to form its designated shape while being pulled and stretched. The extruded profile is then given a water bath and cooled to solidify it.
After cooling, the resulting profile is sturdy and has undergone a meticulous manufacturing process. The final step is quality control and testing, where the profiles are tested for accuracy, analysis, and performance to ensure that they meet all the requirements for a high-quality product.
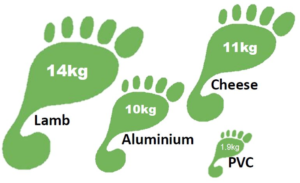
Carbon footprint of PVC compared to other products